Bayesian Seemingly Unrelated Regression Models in High-Dimensional Settings.
BayesSUR
This R package is for high-dimensional multivariate Bayesian variable and covariance selection in linear regression, including methods in Bottolo et al. (2021), Zhao et al. (2021) and Zhao et al. (2024). See the package vignettes BayesSUR.pdf for more information and an additional example below for the BayesSUR model with random effects.
Installation
Install the latest released version from CRAN
install.packages("BayesSUR")
Install the latest development version from GitHub
#install.packages("remotes")
remotes::install_github("mbant/BayesSUR/BayesSUR")
Additional example
The BayesSUR model has been extended to include mandatory variables by assigning Gaussian priors as random effects rather than spike-and-slab priors, named as SSUR-MRF with random effects in Zhao et al. 2023. The R code for the simulated data and real data analyses in Zhao et al. 2023 can be found at the GitHub repository BayesSUR-RE.
Here, we show a simulation example to run the BayesSUR mdoel with random effects.
Simulate data
We design a network as the following figure (a) to construct a complex structure between $20$ response variables and $300$ predictors. It assumes that the responses are divided into six groups, and the first $120$ predictors are divided into nine groups.
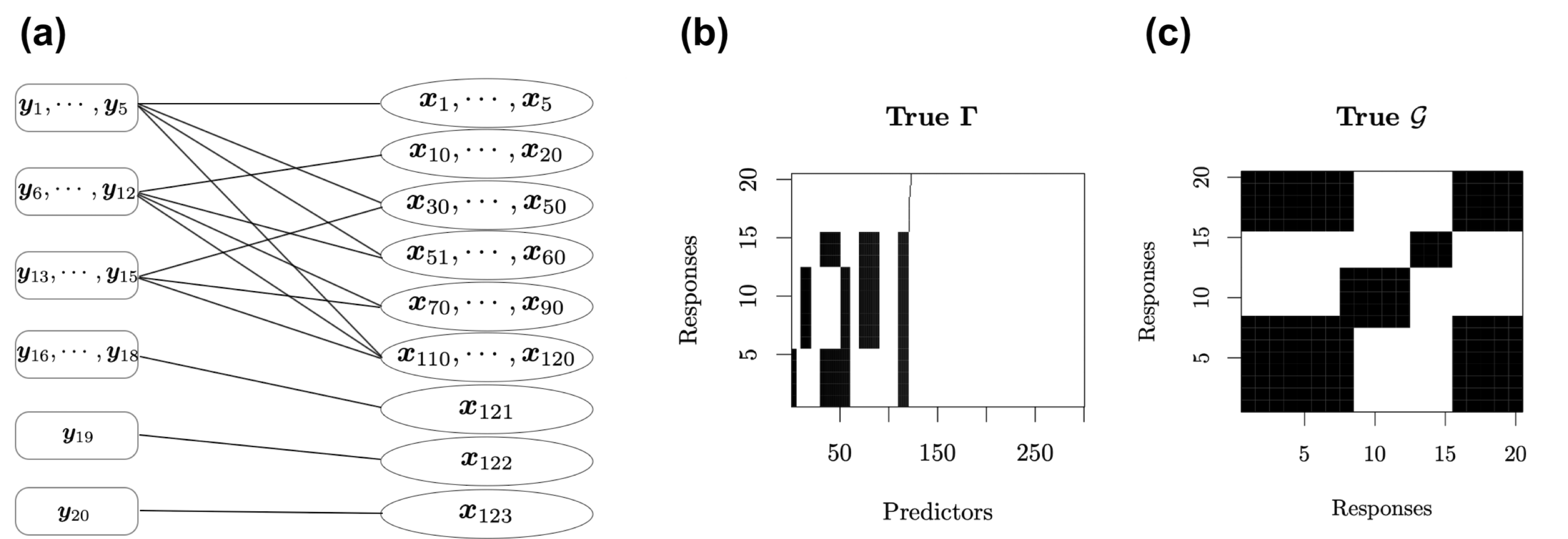
Figure: True relationships between response variables and predictors. (a) Network structure between $\mathbf Y$ and $\mathbf X$. (b) Spare latent indicator variable $\Gamma$ for the associations between $\mathbf Y$ and $\mathbf X$ in the SUR model. Black blocks indicate nonzero coefficients and white blocks indicate zero coefficients. (c) Additional structure in the residual covariance matrix between response variables not explained by $\mathbf X\mathbf B$. Black blocks indicate correlated residuals of the corresponding response variables and white blocks indicate uncorrelated residuals of the corresponding response variables.
Load the simulation function sim.ssur() as follows.
sim.ssur <- function(n, s, p, t0 = 0, seed = 123, mv = TRUE,
t.df = Inf, random.intercept = 0, intercept = TRUE) {
# set seed to fix coefficients
set.seed(7193)
sd_b <- 1
mu_b <- 1
b <- matrix(rnorm((p + ifelse(t0 == 0, 1, 0)) * s, mu_b, sd_b), p + ifelse(t0 == 0, 1, 0), s)
# design groups and pathways of Gamma matrix
gamma <- matrix(FALSE, p + ifelse(t0 == 0, 1, 0), s)
if (t0 == 0) gamma[1, ] <- TRUE
gamma[2:6 - ifelse(t0 == 0, 0, 1), 1:5] <- TRUE
gamma[11:21 - ifelse(t0 == 0, 0, 1), 6:12] <- TRUE
gamma[31:51 - ifelse(t0 == 0, 0, 1), 1:5] <- TRUE
gamma[31:51 - ifelse(t0 == 0, 0, 1), 13:15] <- TRUE
gamma[52:61 - ifelse(t0 == 0, 0, 1), 1:12] <- TRUE
gamma[71:91 - ifelse(t0 == 0, 0, 1), 6:15] <- TRUE
gamma[111:121 - ifelse(t0 == 0, 0, 1), 1:15] <- TRUE
gamma[122 - ifelse(t0 == 0, 0, 1), 16:18] <- TRUE
gamma[123 - ifelse(t0 == 0, 0, 1), 19] <- TRUE
gamma[124 - ifelse(t0 == 0, 0, 1), 20] <- TRUE
G_kron <- matrix(0, s * p, s * p)
G_m <- bdiag(matrix(1, ncol = 5, nrow = 5),
matrix(1, ncol = 7, nrow = 7),
matrix(1, ncol = 8, nrow = 8))
G_p <- bdiag(matrix(1, ncol = 5, nrow = 5), diag(3),
matrix(1, ncol = 11, nrow = 11), diag(9),
matrix(1, ncol = 21, nrow = 21),
matrix(1, ncol = 10, nrow = 10), diag(9),
matrix(1, ncol = 21, nrow = 21), diag(19),
matrix(1, ncol = 11, nrow = 11), diag(181))
G_kron <- kronecker(G_m, G_p)
combn11 <- combn(rep((1:5 - 1) * p, each = length(1:5)) +
rep(1:5, times = length(1:5)), 2)
combn12 <- combn(rep((1:5 - 1) * p, each = length(30:60)) +
rep(30:60, times = length(1:5)), 2)
combn13 <- combn(rep((1:5 - 1) * p, each = length(110:120)) +
rep(110:120, times = length(1:5)), 2)
combn21 <- combn(rep((6:12 - 1) * p, each = length(10:20)) +
rep(10:20, times = length(6:12)), 2)
combn22 <- combn(rep((6:12 - 1) * p, each = length(51:60)) +
rep(51:60, times = length(6:12)), 2)
combn23 <- combn(rep((6:12 - 1) * p, each = length(70:90)) +
rep(70:90, times = length(6:12)), 2)
combn24 <- combn(rep((6:12 - 1) * p, each = length(110:120)) +
rep(110:120, times = length(6:12)), 2)
combn31 <- combn(rep((13:15 - 1) * p, each = length(30:50)) +
rep(30:50, times = length(13:15)), 2)
combn32 <- combn(rep((13:15 - 1) * p, each = length(70:90)) +
rep(70:90, times = length(13:15)), 2)
combn33 <- combn(rep((13:15 - 1) * p, each = length(110:120)) +
rep(110:120, times = length(13:15)), 2)
combn4 <- combn(rep((16:18 - 1) * p, each = length(121)) +
rep(121, times = length(16:18)), 2)
combn5 <- matrix(rep((19 - 1) * p, each = length(122)) +
rep(122, times = length(19)), nrow = 1, ncol = 2)
combn6 <- matrix(rep((20 - 1) * p, each = length(123)) +
rep(123, times = length(20)), nrow = 1, ncol = 2)
combnAll <- rbind(t(combn11), t(combn12), t(combn13),
t(combn21), t(combn22), t(combn23), t(combn24),
t(combn31), t(combn32), t(combn33),
t(combn4), combn5, combn6)
set.seed(seed + 7284)
sd_x <- 1
x <- matrix(rnorm(n * p, 0, sd_x), n, p)
if (t0 == 0 & intercept) x <- cbind(rep(1, n), x)
if (!intercept) {
gamma <- gamma[-1, ]
b <- b[-1, ]
}
xb <- matrix(NA, n, s)
if (mv) {
for (i in 1:s) {
if (sum(gamma[, i]) >= 1) {
if (sum(gamma[, i]) == 1) {
xb[, i] <- x[, gamma[, i]] * b[gamma[, i], i]
} else {
xb[, i] <- x[, gamma[, i]] %*% b[gamma[, i], i]
}
} else {
xb[, i] <- sapply(1:s, function(i) rep(1, n) * b[1, i])
}
}
} else {
if (sum(gamma) >= 1) {
xb <- x[, gamma] %*% b[gamma, ]
} else {
xb <- sapply(1:s, function(i) rep(1, n) * b[1, i])
}
}
corr_param <- 0.9
M <- matrix(corr_param, s, s)
diag(M) <- rep(1, s)
## wanna make it decomposable
Prime <- list(c(1:(s * .4), (s * .8):s),
c((s * .4):(s * .6)),
c((s * .65):(s * .75)),
c((s * .8):s))
G <- matrix(0, s, s)
for (i in 1:length(Prime)) {
G[Prime[[i]], Prime[[i]]] <- 1
}
# check
dimnames(G) <- list(1:s, 1:s)
length(gRbase::mcsMAT(G - diag(s))) > 0
var <- solve(BDgraph::rgwish(n = 1, adj = G, b = 3, D = M))
# change seeds to add randomness on error
set.seed(seed + 8493)
sd_err <- 0.5
if (is.infinite(t.df)) {
err <- matrix(rnorm(n * s, 0, sd_err), n, s) %*% chol(as.matrix(var))
} else {
err <- matrix(rt(n * s, t.df), n, s) %*% chol(as.matrix(var))
}
if (t0 == 0) {
b.re <- NA
z <- NA
y <- xb + err
if (random.intercept != 0) {
y <- y + matrix(rnorm(n * s, 0, sqrt(random.intercept)), n, s)
}
z <- sample(1:4, n, replace = T, prob = rep(1 / 4, 4))
return(list(y = y, x = x, b = b, gamma = gamma, z = model.matrix(~ factor(z) + 0)[, ],
b.re = b.re, Gy = G, mrfG = combnAll))
} else {
# add random effects
z <- t(rmultinom(n, size = 1, prob = c(.1, .2, .3, .4)))
z <- sample(1:t0, n, replace = T, prob = rep(1 / t0, t0))
set.seed(1683)
b.re <- rnorm(t0, 0, 2)
y <- matrix(b.re[z], nrow = n, ncol = s) + xb + err
return(list(
y = y, x = x, b = b, gamma = gamma, z = model.matrix(~ factor(z) + 0)[, ],
b.re = b.re, Gy = G, mrfG = combnAll
))
}
}
To simulate data with sample size $n=250$, responsible variables $s=20$ and covariates $p=300$, we can specify the corresponding parameters in the function sim.ssur() as follows.
library("BayesSUR")
library("Matrix")
n <- 250
s <- 20
p <- 300
sim1 <- sim.ssur(n, s, p, seed = 1)
To simulate data from $4$ individual groups with group indicator variables following the defaul multinomial distribution $multinomial(0.1,0.2,0.3,0.4)$, we can simply add the argument t0 = 4 in the function sim.ssur() as follows.
t0 <- 4
sim2 <- sim.ssur(n, s, p, t0, seed = 1) # learning data
sim2.val <- sim.ssur(n, s, p, t0, seed=101) # validation data
Run BayesSUR model with random effects
According to the guideline of prior specification in Zhao et al. 2023, we first set the following parameters hyperpar and then running the BayesSUR model with random effects via betaPrior = "reGroup" (default betaPrior = "independent" with spike-and-slab priors for all coefficients). For illustration, we run a short MCMC with nIter = 300 and burnin = 100. Note that here the graph used for the Markov random field prior is the true graph from the returned object of the simulation sim2$mrfG.
hyperpar <- list(mrf_d = -2, mrf_e = 1.6, a_w0 = 100, b_w0 = 500, a_w = 15, b_w = 60)
set.seed(1038)
fit2 <- BayesSUR(
data = cbind(sim2$y, sim2$z, sim2$x),
Y = 1:s,
X_0 = s + 1:t0,
X = s + t0 + 1:p,
outFilePath = "sim2_mrf_re",
hyperpar = hyperpar,
gammaInit = "0",
betaPrior = "reGroup",
nIter = 300, burnin = 100,
covariancePrior = "HIW",
standardize = F,
standardize.response = F,
gammaPrior = "MRF",
mrfG = sim2$mrfG,
output_CPO = T
)
## BayesSUR -- Bayesian Seemingly Unrelated Regression Modelling
## Reading input files ... ... successfull!
## Clearing and initialising output files
## Initialising the (SUR) MCMC Chain ... ... DONE!
## Drafting the output files with the start of the chain ... DONE!
##
## Starting 2 (parallel) chain(s) for 300 iterations:
## Temperature ladder updated, new temperature ratio : 1.1
## MCMC ends. --- Saving results and exiting
## Saved to : sim2_mrf_re/data_SSUR_****_out.txt
## Final w0 : 4.9971
## Final w : 2.29497
## Final tau : 0.293487 w/ proposal variance: 1.2229
## Final eta : 0.0471505
## -- Average Omega : 0
## Final temperature ratio : 1.1
##
## DONE, exiting!
Check some summarized information of the results:
summary(fit2)
## Call:
## BayesSUR(data = cbind(sim2$y, sim2$z, sim2$x), ...)
##
## CPOs:
## Min. 1st Qu. Median 3rd Qu. Max.
## 0.0001896996 0.0242732651 0.0348570615 0.0465600279 0.1312571329
##
## Number of selected predictors (mPIP > 0.5): 19 of 20x300
##
## Top 10 predictors on average mPIP across all responses:
## X.74 X.69 X.77 X.82 X.114 X.116 X.122 X.157 X.265
## 0.081840 0.049500 0.049500 0.049500 0.049500 0.049500 0.049500 0.049500 0.049500
## X.16
## 0.047015
##
## Top 10 responses on average mPIP across all predictors:
## X.8 X.10 X.5 X.19 X.11 X.9 X.6
## 0.012703000 0.010049000 0.007943333 0.006600000 0.005522333 0.004029667 0.003001667
## X.12 X.2 X.4
## 0.002852333 0.002769667 0.002404667
##
## Expected log pointwise predictive density (elpd) estimates:
## elpd.LOO = -16803.49, elpd.WAIC = -16799.94
##
## MCMC specification:
## iterations = 300, burn-in = 100, chains = 2
## gamma local move sampler: bandit
## gamma initialisation: 0
##
## Model specification:
## covariance prior: HIW
## gamma prior: MRF
##
## Hyper-parameters:
## a_w b_w nu a_tau b_tau a_eta b_eta mrf_d mrf_e a_w0 b_w0
## 15.0 60.0 22.0 0.1 10.0 0.1 1.0 -2.0 1.6 100.0 500.0
Show the estimates of regression coefficients, variable selection indicators and residual graph
# show estimates
plot(fit2, estimator=c("beta","gamma","Gy"), type="heatmap", name.predictors = "auto")
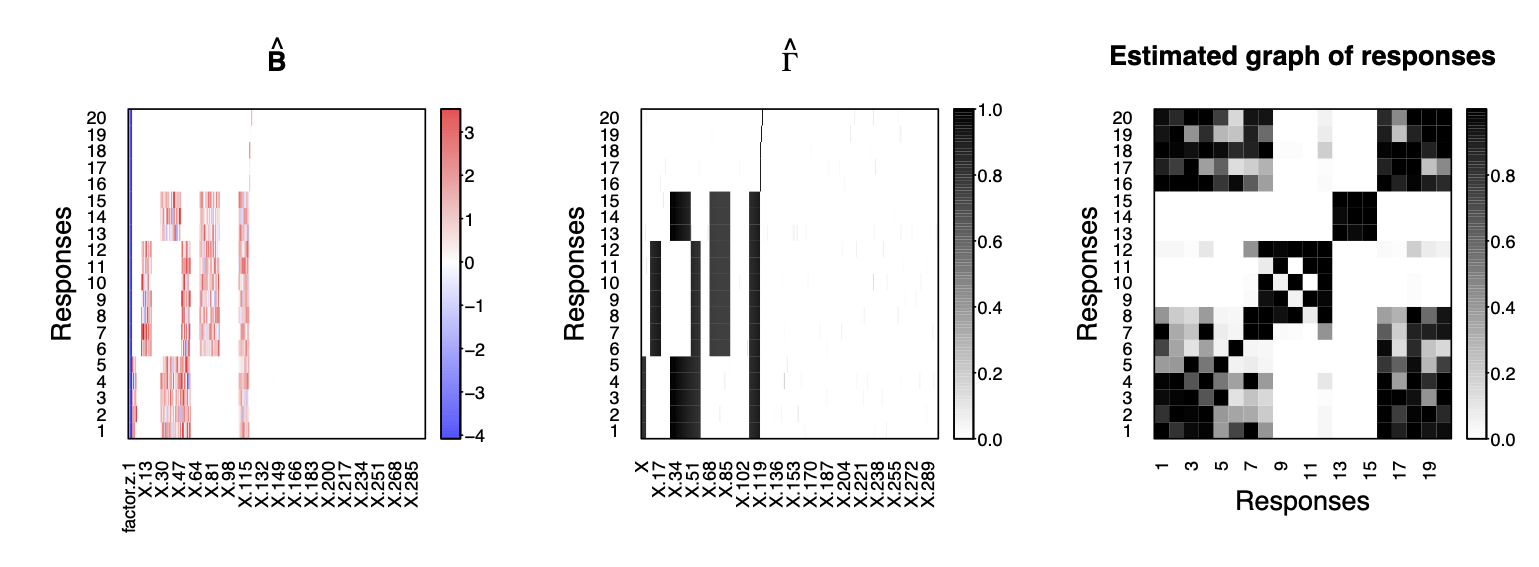
Compute the model performace with respect to variable selection
# compute accuracy, sensitivity, specificity of variable selection
gamma <- getEstimator(fit2)
(accuracy <- sum(data.matrix(gamma > 0.5) == sim2$gamma) / prod(dim(gamma)))
## [1] 0.8725
(sensitivity <- sum((data.matrix(gamma > 0.5) == 1) & (sim2$gamma == 1)) / sum(sim2$gamma == 1))
## [1] 0.01558442
(specificity <- sum((data.matrix(gamma > 0.5) == 0) & (sim2$gamma == 0)) / sum(sim2$gamma == 0))
## [1] 0.9986616
Compute the model performance with respect to response prediction
# compute RMSE and RMSPE for prediction performance
beta <- getEstimator(fit2, estimator = "beta", Pmax = .5, beta.type = "conditional")
(RMSE <- sqrt(sum((sim2$y - cbind(sim2$z, sim2$x) %*% beta)^2) / prod(dim(sim2$y))))
## [1] 8.723454
(RMSPE <- sqrt(sum((sim2.val$y - cbind(sim2.val$z, sim2.val$x) %*% beta)^2) / prod(dim(sim2.val$y))))
## [1] 8.859939
Compute the model performance with respect to coefficient bias
# compute bias of beta estimates
b <- sim2$b
b[sim2$gamma == 0] <- 0
(beta.l2 <- sqrt(sum((beta[-c(1:4), ] - b)^2) / prod(dim(b))))
## [1] 0.5039502
Compute the model performance with respect to covariance selection
g.re <- getEstimator(fit2, estimator = "Gy")
(g.accuracy <- sum((g.re > 0.5) == sim2$Gy) / prod(dim(g.re)))
## [1] 0.585
(g.sensitivity <- sum(((g.re > 0.5) == sim2$Gy)[sim2$Gy == 1]) / sum(sim2$Gy == 1))
## [1] 0.2475248
(g.specificity <- sum(((g.re > 0.5) == sim2$Gy)[sim2$Gy == 0]) / sum(sim2$Gy == 0))
## [1] 0.9292929
References
Leonardo Bottolo, Marco Banterle, Sylvia Richardson, Mika Ala-Korpela, Marjo-Riitta Järvelin, Alex Lewin (2021). A computationally efficient Bayesian seemingly unrelated regressions model for high-dimensional quantitative trait loci discovery. Journal of the Royal Statistical Society: Series C (Applied Statistics), 70(4):886-908. DOI: 10.1111/rssc.12490.
Zhi Zhao, Marco Banterle, Leonardo Bottolo, Sylvia Richardson, Alex Lewin, Manuela Zucknick (2021). BayesSUR: An R package for high-dimensional multivariate Bayesian variable and covariance selection in linear regression. Journal of Statistical Software, 100(11):1-32. DOI: 10.18637/jss.v100.i11.
Zhi Zhao, Marco Banterle, Alex Lewin, Manuela Zucknick (2023). Multivariate Bayesian structured variable selection for pharmacogenomic studies. Journal of the Royal Statistical Society: Series C (Applied Statistics), 73(2):420-443 qlad102. DOI: 10.1093/jrsssc/qlad102.

