Time to Event Outcome in Experimental Designs of Pre-Clinical Studies.
PDXpower
The PDXpower package can conduct power analysis for time-to-event outcome based on empirical simulations.
Installation
You can install the development version of PDXpower from GitHub with:
# install.packages("devtools")
devtools::install_github("shanpengli/PDXpower")
Example
Below is a toy example how to conduct power analysis based on a preliminary dataset animals1. Particularly, we need to specify a formula that fits a ANOVA mixed effects model with correlating variables in animals1, where ID is the PDX line number, Y is the event time variable, and Tx is the treatment variable.
Next, run power analysis by fitting a ANOVA mixed effects model on animals1.
library(PDXpower)
#> Loading required package: survival
#> Loading required package: parallel
data(animals1)
### Power analysis on a preliminary dataset by assuming the time to event is log-normal
PowTab <- PowANOVADat(data = animals1, formula = log(Y) ~ Tx,
random = ~ 1|ID, n = c(3, 5, 10), m = c(2, 3, 4), sim = 100)
#> Parameter estimates based on the pilot data:
#> Treatment effect (beta): 0.7299
#> Variance of random effect (tau2): 0.0332
#> Random error variance (sigma2): 0.386
#>
#> Monte Carlo power estimate, calculated as the
#> proportion of instances where the null hypothesis
#> H_0: beta = 0 is rejected (n = number of PDX lines,
#> m = number of animals per arm per PDX line,
#> N = total number of animals for a given combination of n and m):
#> n m N Power (%)
#> 1 3 2 12 43
#> 2 3 3 18 65
#> 3 3 4 24 77
#> 4 5 2 20 70
#> 5 5 3 30 87
#> 6 5 4 40 95
#> 7 10 2 40 98
#> 8 10 3 60 99
#> 9 10 4 80 100
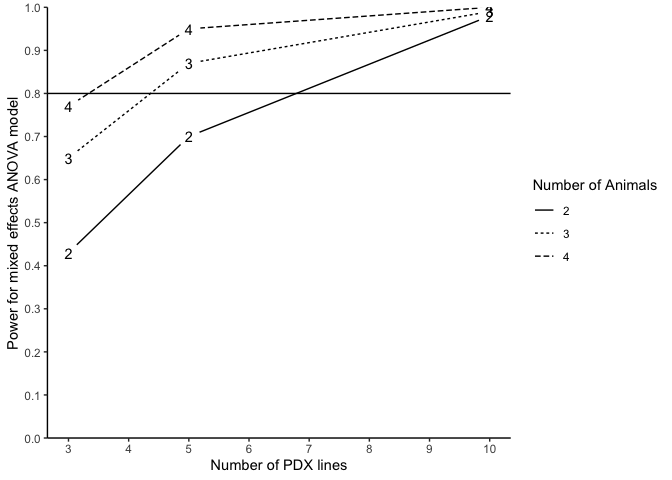
The following code generates a power curve based on the object PowTab.
plotpower(PowTab[[4]], ylim = c(0, 1))
Or we can fit a ANOVA fixed effect model for running power analysis.
### Power analysis by specifying the median survival
### of control and treatment group and assuming
### the time-to-event is log-normal distributed
PowTab <- PowANOVA(ctl.med.surv = 2.4, tx.med.surv = 7.2, icc = 0.1, sigma2 = 1, sim = 100, n = c(3, 5, 10), m = c(2, 3, 4))
#> Treatment effect (beta): -1.098612
#> Variance of random effect (tau2): 0.1111111
#> Intra-PDX correlation coefficient (icc): 0.1
#> Random error variance (sigma2): 1
#>
#> Monte Carlo power estimate, calculated as the
#> proportion of instances where the null hypothesis
#> H_0: beta = 0 is rejected (n = number of PDX lines,
#> m = number of animals per arm per PDX line,
#> N = total number of animals for a given combination
#> of n and m):
#> n m N Power (%)
#> 1 3 2 12 37
#> 2 3 3 18 56
#> 3 3 4 24 85
#> 4 5 2 20 65
#> 5 5 3 30 80
#> 6 5 4 40 97
#> 7 10 2 40 93
#> 8 10 3 60 99
#> 9 10 4 80 100
Alternatively, one can run power analysis by fitting a Cox frailty model. Here we present another dataset animals2. Particularly, we need to specify a formula that fits a Cox frailty model with correlating variables in animals2, where ID is the PDX line number, Y is the event time variable, Tx is the treatment variable, and status is the event status.
data(animals2)
### Power analysis on a preliminary dataset by assuming the time to event is Weibull-distributed
PowTab <- PowFrailtyDat(data = animals2, formula = Surv(Y, status) ~ Tx + cluster(ID),
n = c(3, 5, 10), m = c(2, 3, 4), sim = 100)
#> Parameter estimates based on the pilot data:
#> Scale parameter (lambda): 0.0154
#> Shape parameter (nu): 2.1722
#> Treatment effect (beta): -0.8794
#> Variance of random effect (tau2): 0.0422
#>
#> Monte Carlo power estimate, calculated as the
#> proportion of instances where the null hypothesis
#> H_0: beta = 0 is rejected (n = number of PDX lines,
#> m = number of animals per arm per PDX line,
#> N = total number of animals for a given combination
#> of n and m,
#> Censoring Rate = average censoring rate across 500
#> Monte Carlo samples):
#> n m N Power (%) for Cox's frailty Censoring Rate
#> 1 3 2 12 36.49 0
#> 2 3 3 18 42.35 0
#> 3 3 4 24 62.22 0
#> 4 5 2 20 49.30 0
#> 5 5 3 30 69.23 0
#> 6 5 4 40 78.72 0
#> 7 10 2 40 90.57 0
#> 8 10 3 60 88.78 0
#> 9 10 4 80 96.91 0
PowTab
#> $lambda
#> [1] 0.01540157
#>
#> $nu
#> [1] 2.172213
#>
#> $beta
#> Tx
#> -0.879356
#>
#> $tau2
#> [1] 0.04224566
#>
#> $PowTab
#> n m N Power (%) for Cox's frailty Censoring Rate
#> 1 3 2 12 36.49 0
#> 2 3 3 18 42.35 0
#> 3 3 4 24 62.22 0
#> 4 5 2 20 49.30 0
#> 5 5 3 30 69.23 0
#> 6 5 4 40 78.72 0
#> 7 10 2 40 90.57 0
#> 8 10 3 60 88.78 0
#> 9 10 4 80 96.91 0
#>
#> attr(,"class")
#> [1] "PowFrailtyDat"
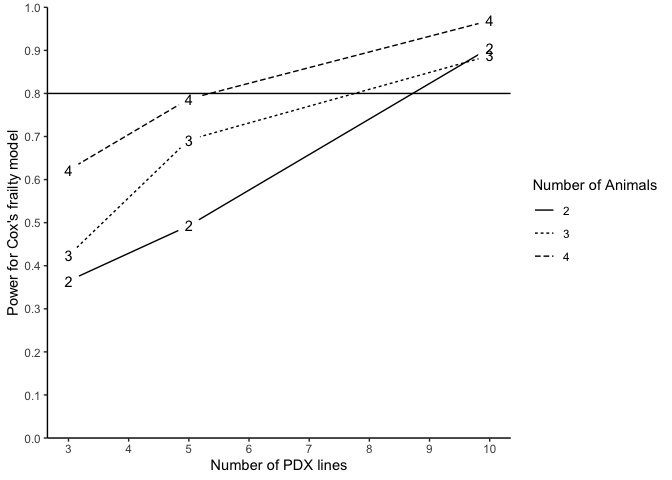
The following code generates a power curve based on the object PowTab.
plotpower(PowTab[[5]], ylim = c(0, 1))
Alternatively, we may also conduct power analysis based on median survival of two randomized arms. We suppose that the median survival of the control and treatment arm is 2.4 and 4.8, allowing a PDX line has 10% marginal error (tau2=0.1) of treatment effect and an exponential event time, a power analysis may be done as below:
### Assume the time to event outcome is weibull-distributed
PowTab <- PowFrailty(ctl.med.surv = 2.4, tx.med.surv = 4.8, nu = 1, tau2 = 0.1, sim = 100,
n = c(3, 5, 10), m = c(2, 3, 4))
#> Treatment effect (beta): -0.6931472
#> Scale parameter (lambda): 0.2888113
#> Shape parameter (nu): 1
#> Variance of random effect (tau2): 0.1
#>
#> Monte Carlo power estimate, calculated as the
#> proportion of instances where the null hypothesis
#> H_0: beta = 0 is rejected (n = number of PDX lines,
#> m = number of animals per arm per PDX line,
#> N = total number of animals for a given combination
#> of n and m,
#> Censoring Rate = average censoring rate across 500
#> Monte Carlo samples):
#> n m N Power (%) for Cox's frailty Censoring Rate
#> 1 3 2 12 22.45 0
#> 2 3 3 18 21.05 0
#> 3 3 4 24 41.41 0
#> 4 5 2 20 35.16 0
#> 5 5 3 30 44.79 0
#> 6 5 4 40 62.89 0
#> 7 10 2 40 67.01 0
#> 8 10 3 60 73.74 0
#> 9 10 4 80 86.73 0