Stratified Medicine.
StratifiedMedicine
The goal of StratifiedMedicine is to develop analytic and visualization tools to aid in stratified and personalized medicine. Stratified medicine aims to find subsets or subgroups of patients with similar treatment effects, for example responders vs non-responders, while personalized medicine aims to understand treatment effects at the individual level (does a specific individual respond to treatment A?). Development of the package is ongoing.
Currently, the main tools in this package area: (1) Filter Models (identify important variables and reduce input covariate space), (2) Patient-Level Estimate Models (using regression models, estimate counterfactual quantities, such as the conditional average treatment effect or CATE), (3) Subgroup Models (identify groups of patients using tree-based approaches), and (4) Parameter Estimation (across the identified subgroups), and (5) PRISM (Patient Response Identifiers for Stratified Medicine; combines tools 1-4). Development of this package is ongoing.
Given a data-structure of (Y,A,X) (outcome, treatments, covariates), PRISM is a four step feature, which comprise of individual tools mentioned above:
Filter (filter_train): Reduce covariate space by removing variables unrelated to outcome/treatment.
Patient-level estimate (ple_train): Estimate counterfactual patient-level quantities, for example the conditional average treatment effect (CATE), E(Y|X=x, A=1)-E(Y|X=x,A=0).
Subgroup model (submod_train): Tree-based models to identify groups with heterogeneous treatment effects. Based on initial subgroups (ex: tree nodes), we may also pool patients into “benefitting” and “non-benefitting” (see “pool” argument). With or without pooling, subgroup-specific treatment estimates and variability metrics are also provided.
Resampling: Steps 1-3 are repeated through bootstrap resampling for improved statistical performance of subgroup-specific estimates.
Installation
You can install the released version of StratifiedMedicine from CRAN with:
install.packages("StratifiedMedicine")
And the development version from GitHub with:
# install.packages("devtools")
devtools::install_github("thomasjemielita/StratifiedMedicine")
Example: Continuous Outcome with Binary Treatment
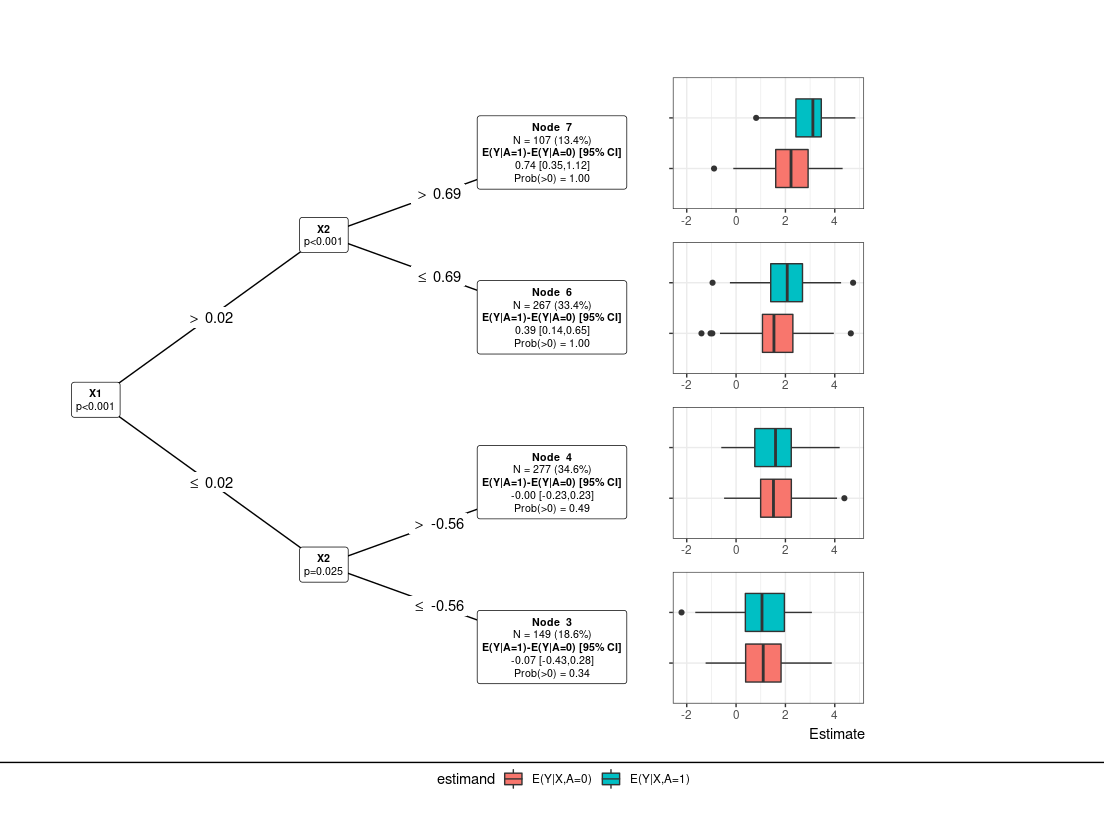
Suppose the estimand or question of interest is the average treatment effect, E(Y|A=1)-E(Y|A=0). The goal is to understand whether there is any treatment heterogeneity across patients and if there are any distinct subgroups with similar responses. In this example, we simulate continuous data where roughly 30% of the patients receive no treatment-benefit for using A=1 (study treatment) vs A=0 (control). Responders (should receive study treatment) vs non-responders (should receive control) are defined by the continuous predictive covariates X1 and X2 for a total of four subgroups. Subgroup treatment effects are 0 (X1 \<= 0, X2 \<= 0), 0.25 (X1 \> 0, X2 \<= 0), 0.45 (X1 \<= 0, X2 \> 0), 0.65 (X1 > 0, X2 >0)
library(StratifiedMedicine)
## basic example code
dat_ctns = generate_subgrp_data(family="gaussian")
Y = dat_ctns$Y
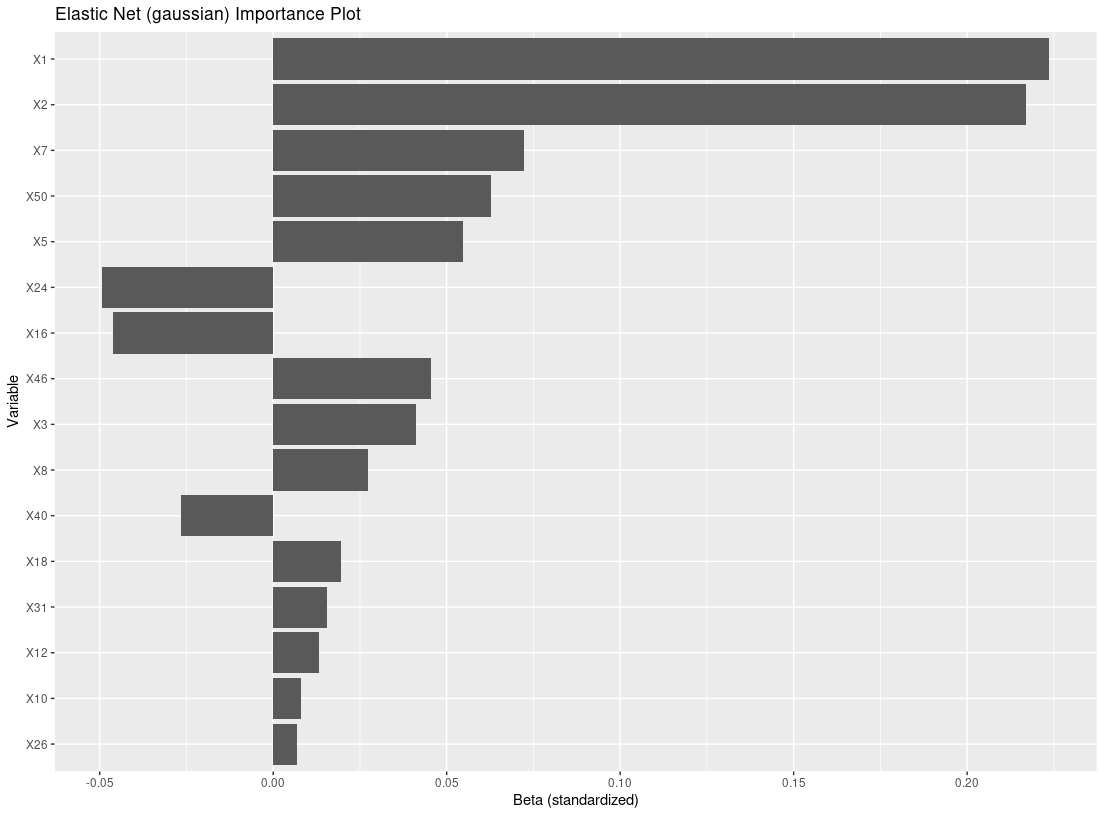
X = dat_ctns$X # 50 covariates, 46 are noise variables, X1 and X2 are truly predictive
A = dat_ctns$A # binary treatment, 1:1 randomized
# Filter #
res_f <- filter_train(Y, A, X, filter="glmnet")
plot_importance(res_f)
# counterfactual estimates (ple) #
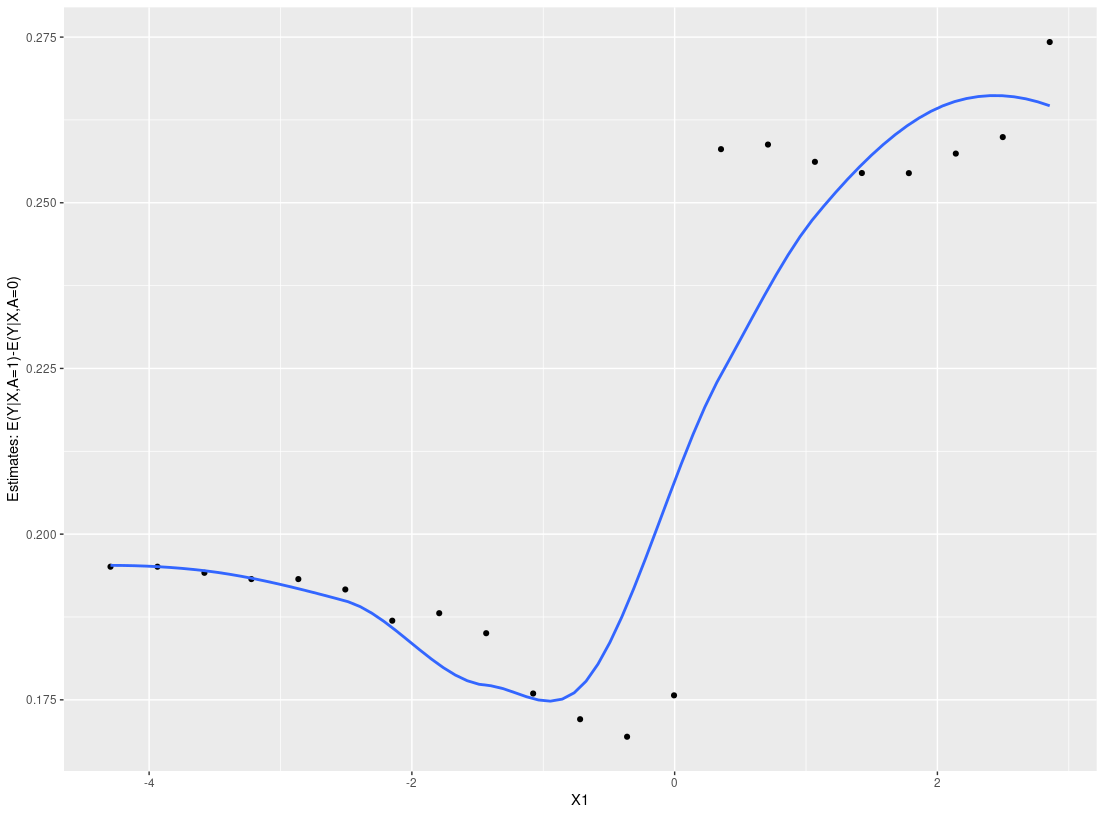
res_p <- ple_train(Y, A, X, ple="ranger")
plot_dependence(res_p, X=X, vars="X1")
#> `geom_smooth()` using method = 'loess' and formula 'y ~ x'
# subgroup model #
res_s <- submod_train(Y, A, X, submod="lmtree")
plot_tree(res_s)
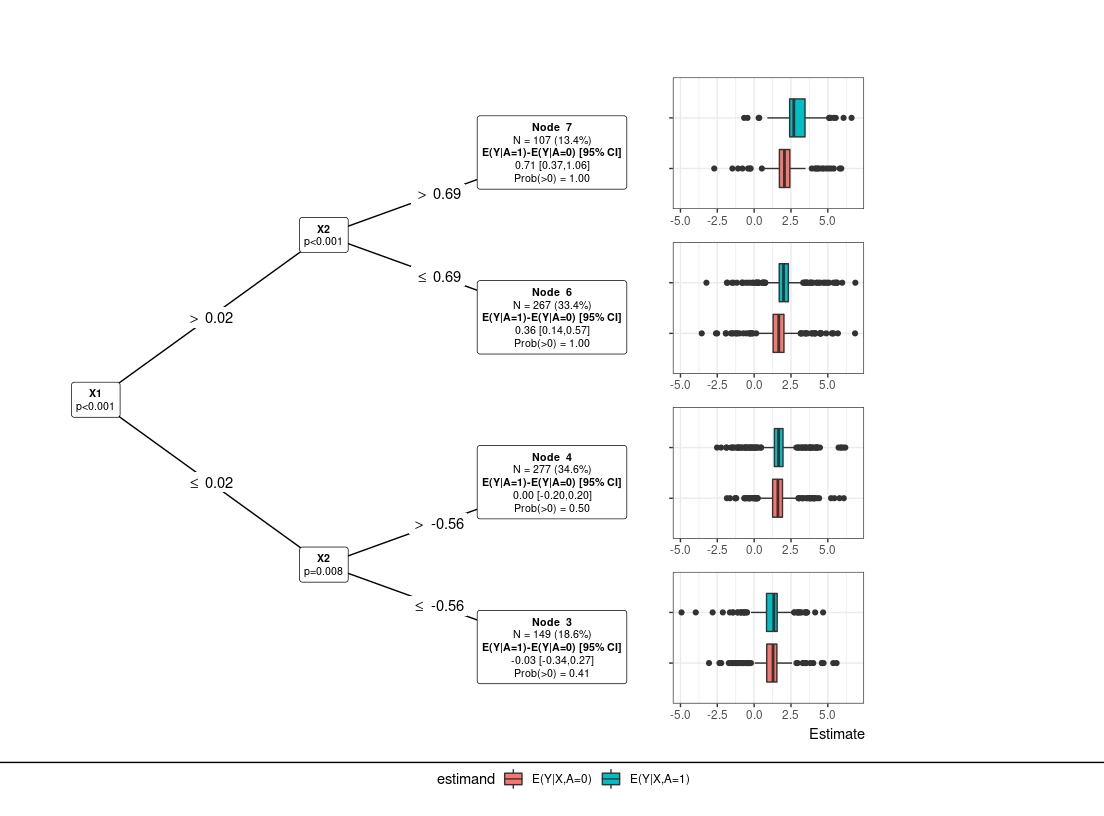
# PRISM Default: filter=glmnet, ple=ranger, submod=lmtree, param=dr #
res0 = PRISM(Y=Y, A=A, X=X, submod.hyper = list(maxdepth = 3))
#> Observed Data
#> Filtering: glmnet
#> Counterfactual Estimation: ranger (X-learner)
#> Subgroup Identification: lmtree
#> Treatment Effect Estimation: dr
plot(res0) # default: tree plot
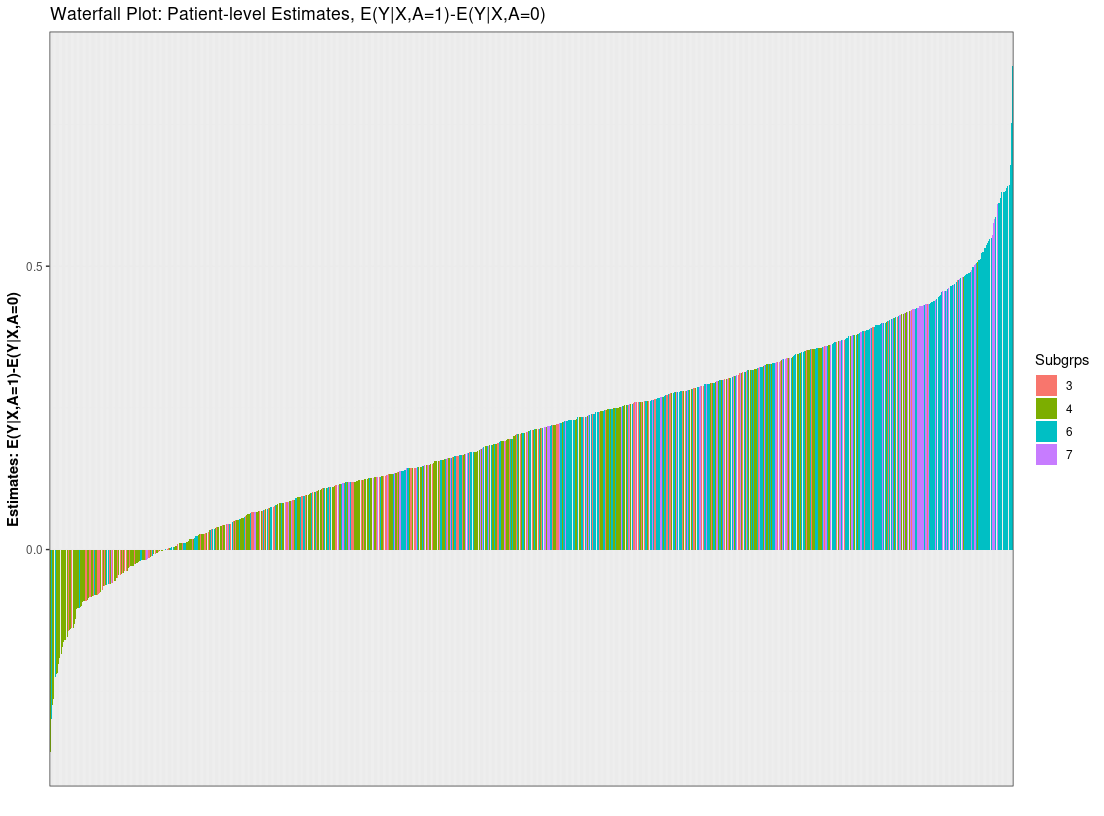
plot(res0, type="PLE:waterfall")
## Dependence Plots (univariate and heat maps)
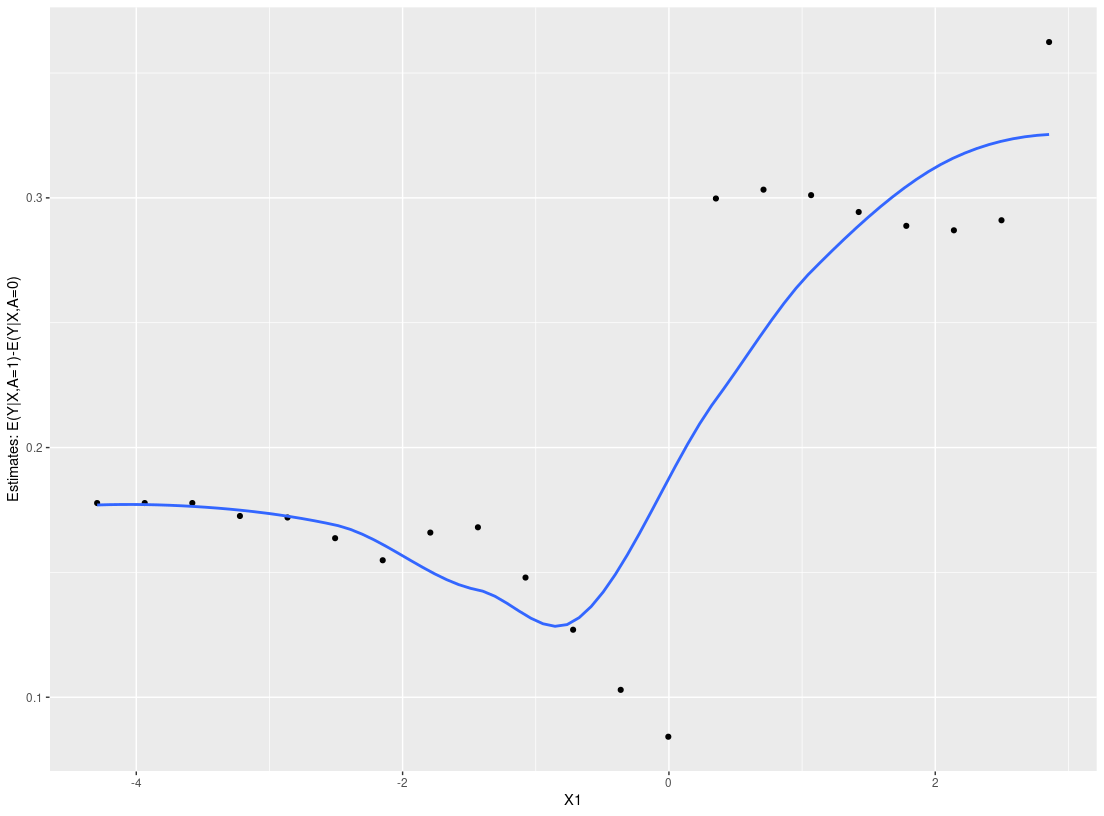
plot_dependence(res0, vars="X1")
#> `geom_smooth()` using method = 'loess' and formula 'y ~ x'
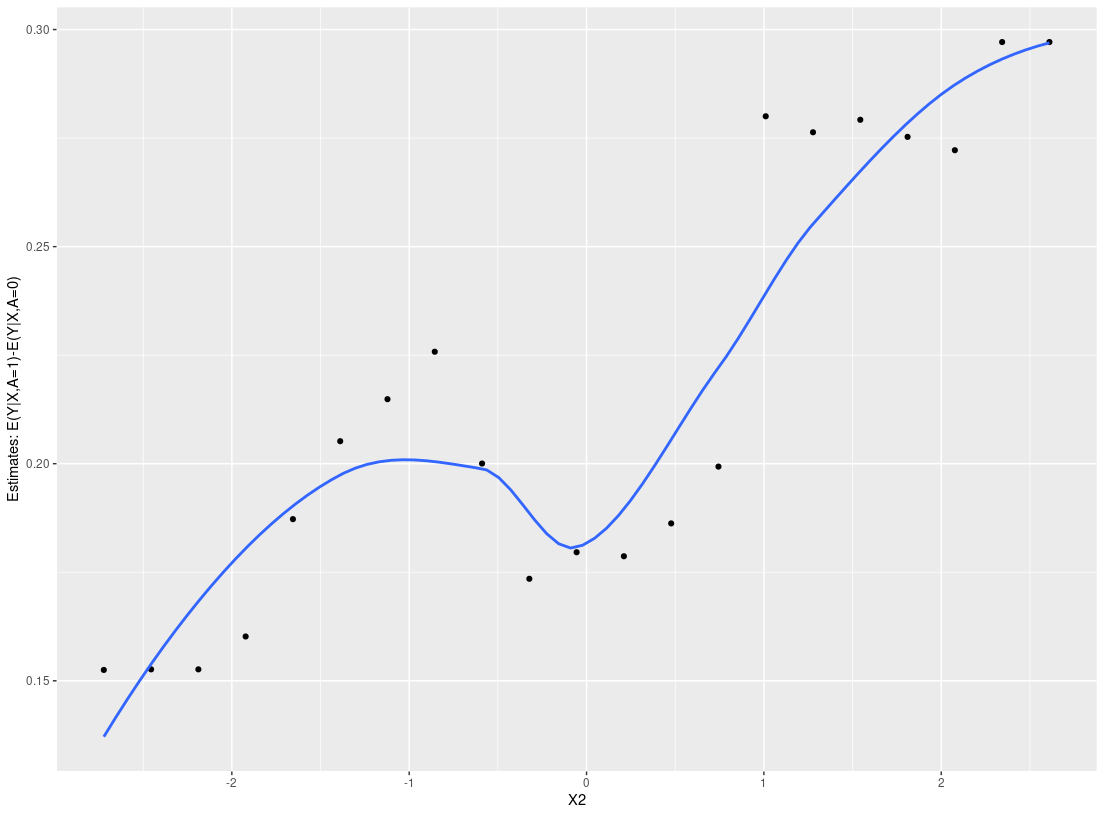
plot_dependence(res0, vars="X2")
#> `geom_smooth()` using method = 'loess' and formula 'y ~ x'
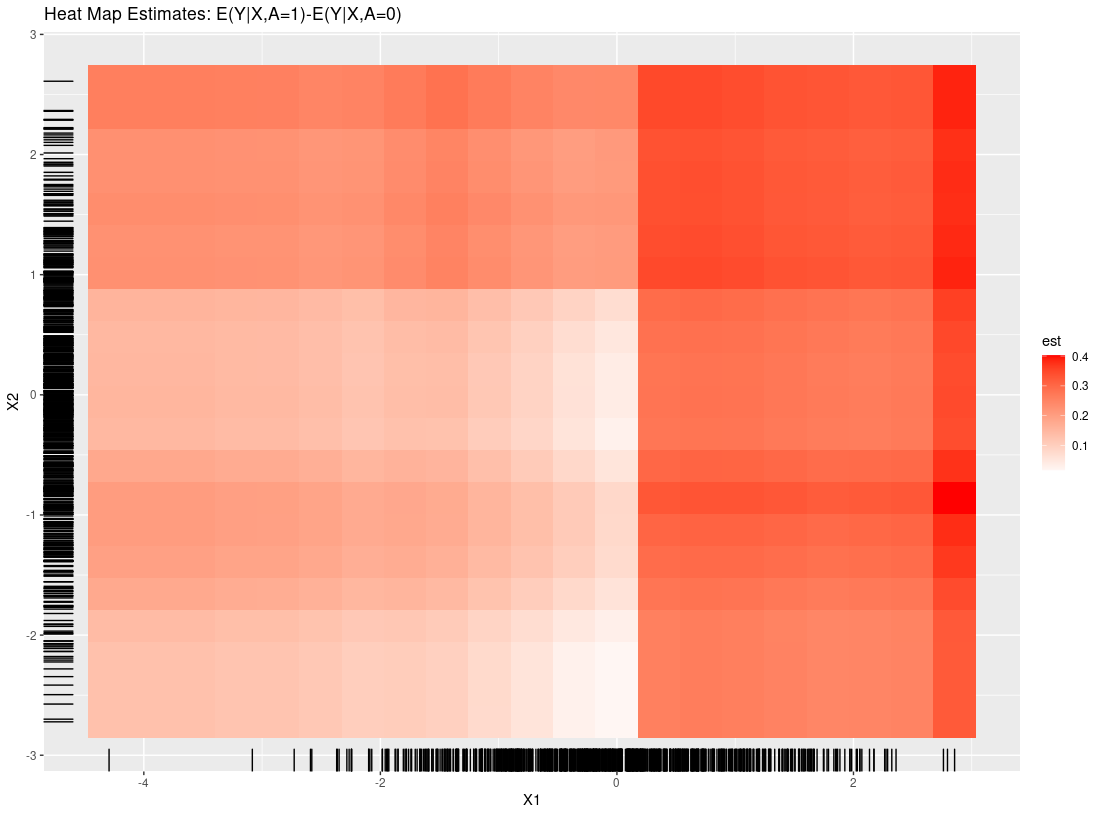
plot_dependence(res0, vars=c("X1", "X2"))
Overall, the StratifiedMedicine R package provides information at the patient-level and the subgroup-level (if any). While there are defaults in place, the user can also input their own functions/model wrappers into each of the individual tools. For more details and more examples, we refer the reader to the following vignettes, Overview of Package, User Specific Models.