Spatial Subsampling of Biodiversity Occurrence Data.
divvy

Divvy up diversity into geographic regions of fair comparison!
Overview
An R package offering spatial subsampling functions for biogeography and biodiversity studies, whether analysing fossil or modern taxon occurrence data, as described by Antell et al. (2023), ‘Spatial standardization of taxon occurrence data—a call to action’. A preprint of the Paleobiology paper is available at Earth ArXiv.
Three types of subsampling are available to to standardise the number and maximum spread (dispersion) of sites within a region of specified size:
cookies: Imposes a radial constraint on the spatial bounds of a subsample and standardises area by rarefying the number of localitiesclustr: Aggregates sites that are nearest neighbours (connecting them with a minimum spanning tree) to impose a maximum diameter on the spatial bounds of a subsample, and optionally rarefies localitiesbandit: Rarefies the number of localities within bands of equal latitude
Additional functions include uniqify to subset an occurrence dataset to unique taxon-coordinate combinations, sdSumry to calculate basic spatial coverage and diversity metadata for a dataset or its subsamples, rangeSize to calculate five measures of geographic range size, and classRast to generate a raster containing the most common environment or trait for point occurrences falling in each grid cell.
There are vignettes accompanying the package, to demonstrate use cases of divvy functions and describe common considerations for analyses of taxonomic occurrence data. You can view the compiled documents at the package website under ‘Getting started’.
Installation
You can install the newest release of divvy from CRAN:
install.packages('divvy')
Alternatively, you can install the development version of divvy from GitHub with help from devtools:
# install.packages('devtools')
devtools::install_github('GawainAntell/divvy')
Usage examples
bivalves: Package data
(Palaeo)ecologists often want to inspect basic information about taxon occurrence datasets such as number of occurrences, number of unique localities, general size and position of the study region, and biodiversity. This may be an initial step to become acquainted with the data, or it may be a final step of analysis to estimate ecological variables of interest. Let’s load one of the example datasets in with divvy to demonstrate some functions that may help analyse it. The occurrences are latitude-longitude point coordinates of Pliocene bivalves from the Paleobiology Database; before proceeding further, let’s rasterise these into an equal-area grid, a common starting point for biogeography analysis.
library(divvy)
data('bivalves')
# initialise Equal Earth projected coordinates
library(terra)
rWorld <- rast()
prj <- 'EPSG:8857'
rPrj <- project(rWorld, prj, res = 200000) # 200,000m is approximately 2 degrees
values(rPrj) <- 1:ncell(rPrj)
# coordinate column names for the current and target coordinate reference system
xyCartes <- c('paleolng','paleolat')
xyCell <- c('cellX','cellY')
# extract cell number and centroid coordinates associated with each occurrence
llOccs <- vect(bivalves, geom = xyCartes, crs = 'epsg:4326')
prjOccs <- project(llOccs, prj)
bivalves$cell <- cells(rPrj, prjOccs)[,'cell']
bivalves[, xyCell] <- xyFromCell(rPrj, bivalves$cell)
uniqify: Subset to unique occurrences
Now let’s examine the data with some divvy functions. First, we can apply uniqify to leave out any duplicate occurrences of a taxon within a grid cell. This shortens the dataset (and thereby reduces memory use) by more than half.
nrow(bivalves)
#> [1] 8095
bivalves <- uniqify(bivalves, taxVar = 'genus', xy = xyCell)
nrow(bivalves)
#> [1] 3061
sdSumry: Summary spatial and diversity metrics
How many taxa are there? Over how many sites (equal-area grid cells)? How many degrees of latitude do those sites span? The sdSumry function returns this and related spatial and diversity metadata.
sdSumry(bivalves, taxVar = 'genus', xy = xyCell, crs = prj)
#> nOcc nLoc centroidX centroidY latRange greatCircDist meanPairDist
#> [1,] 3061 157 -434404.9 1647720 137.4159 28917.12 11375.07
#> minSpanTree SCOR nTax
#> [1,] 93349.91 20.64401 550
There are just over 3000 unique taxon-site occurrences, including 550 genera from 157 grid cells across 137 degrees latitude.
rangeSize: Calculate geographic range size
Maybe we aren’t interested in community ecology and instead care about the geographic distribution of focal taxa, such as the mussel Mytilus and scallop Yabepecten. Provide the coordinates for these two taxa to divvy’s rangeSize function:
myti <- bivalves[bivalves$genus == 'Mytilus', xyCell]
yabe <- bivalves[bivalves$genus == 'Yabepecten', xyCell]
rangeSize(myti, crs = prj)
#> nLoc centroidX centroidY latRange greatCircDist meanPairDist minSpanTree
#> [1,] 18 67152.05 4750551 99.31458 22351.73 10390.96 37303.68
rangeSize(yabe, crs = prj)
#> nLoc centroidX centroidY latRange greatCircDist meanPairDist minSpanTree
#> [1,] 2 11856041 5039440 1.808585 200 200 200
Mytilus is observed in 18 grid cells spread over tens of thousands of kilometers. In contrast, Yabepecten occurs in only two localities, 200 km apart at their grid cell centroids.
If we back-transform the two locality’s coordinates from Equal Earth projection to familiar latitude-longitude, we can tell Yabepecten has been reported only in northern Honshu, Japan (140-141 E longitude, 40-42 N latitude).
yabeVect <- vect(yabe, geom = xyCell, crs = prj)
project(yabeVect, 'epsg:4326')
#> class : SpatVector
#> geometry : points
#> dimensions : 2, 0 (geometries, attributes)
#> extent : 139.6679, 141.3001, 40.16519, 41.97377 (xmin, xmax, ymin, ymax)
#> coord. ref. : lon/lat WGS 84 (EPSG:4326)
cookies: Subsample global data into equivalent regions
The bivalves dataset spans most of the world’s oceans. If we were interested in a question such as how diversity at this Pliocene time step compares against diversity from an earlier interval when geographic sampling coverage was much more limited, we’d have to account for this different data distribution—otherwise, we’d unfairly estimate the well-sampled Pliocene to be far more diverse. Geographic standardisation of both area (acreage or number of sites studied) and dispersion (amount sites are spread apart) allows fair comparisons of ecological variables like richness, whether between time steps, environments, or other comparison groups with heterogeneous spatial coverage.
divvy offers three functions for subsampling (cookies, clustr, and bandit) which differ in how they standardise for dispersion. The easiest form of subsampling to visualise is applying a circular constraint to define the bounds of a subsample region. Within that region, a given number of sites are selected, to standardise area. Here, let’s take 10 subsamples of the bivalves occurrences, each containing 12 sites within a circular region of 1500km (about the size of Australia.)
set.seed(1)
circLocs <- cookies(dat = bivalves, xy = xyCell,
iter = 10, nSite = 12, r = 1500,
crs = prj, output = 'full')
Below is a map of one possible subsample (red, with regional constraint drawn around it). Sites not included in the subsample, including one within the regional constraint, are plotted in blue.
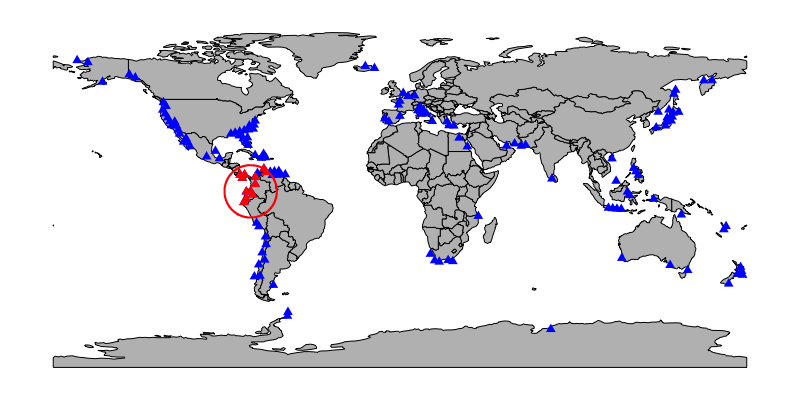
An individual subsample (one data.frame element of the returned list object) contains all occurrences from the subsampled sites. Here’s a peek at the first subsample, to show it’s just a subset of bivalves.
str(circLocs[[1]])
#> 'data.frame': 338 obs. of 12 variables:
#> $ genus : chr "Anomia" "Chlamys" "Mercenaria" "Ostrea" ...
#> $ paleolng : num -80.5 -80.5 -80.5 -80.5 -87.4 ...
#> $ paleolat : num 27.5 27.5 27.5 27.5 31 ...
#> $ collection_no: int 41496 41496 41496 41496 41824 58893 58893 58893 58893 58893 ...
#> $ reference_no : int 11119 11119 11119 11119 11118 16700 16700 16700 16700 16700 ...
#> $ environment : chr "marine indet." "marine indet." "marine indet." "marine indet." ...
#> $ max_ma : num 5.33 5.33 5.33 5.33 5.33 ...
#> $ min_ma : num 3.6 3.6 3.6 3.6 3.6 ...
#> $ accepted_name: chr "Anomia simplex" "Chlamys" "Mercenaria campechiensis" "Ostrea" ...
#> $ cell : num 4178 4178 4178 4178 3832 ...
#> $ cellX : num -7343959 -7343959 -7343959 -7343959 -7743959 ...
#> $ cellY : num 3539440 3539440 3539440 3539440 3939440 ...
Because each subsample has the same information structure as the original dataset, we can analogously calculate summary spatial and diversity data for them using sdSumry. Each row of the returned matrix corresponds to one of the ten subsamples. In this small group of replicate subsamples, regional richness ranges from 117 to 194 genera. The number of localities is always 12, as was specified above, and the dispersion of subsamples is always within the upper bound set by the diameter of 3,000km (e.g. maximum great circle distance across any subsample’s sites is 2807km).
sdSumry(circLocs, taxVar = 'genus', xy = xyCell, crs = prj)
#> nOcc nLoc centroidX centroidY latRange greatCircDist meanPairDist
#> 1 338 12 -6977292.4 4156106 10.25901 1612.452 781.6607
#> 2 265 12 -10160625.7 4072773 17.14477 2039.608 910.6453
#> 3 410 12 -6727292.4 2356106 17.67042 2720.294 1289.9956
#> 4 252 12 -10127292.4 3889440 18.77490 2209.072 888.0877
#> 5 460 12 -6993959.1 4056106 11.91278 1720.465 757.5157
#> 6 446 12 406040.9 5589440 11.37344 1562.050 833.9107
#> 7 293 12 -10177292.4 4239440 20.61302 2408.319 866.7620
#> 8 224 12 -10127292.4 5239440 27.00700 2807.134 1119.3347
#> 9 446 12 406040.9 5589440 11.37344 1562.050 833.9107
#> 10 350 12 256040.9 5389440 14.91345 1811.077 1047.1642
#> minSpanTree SCOR nTax
#> 1 2814.214 33.87300 141
#> 2 2800.000 26.46397 121
#> 3 4097.770 40.52424 194
#> 4 2847.214 25.14927 117
#> 5 2731.371 48.65573 166
#> 6 3402.094 44.22359 190
#> 7 2965.685 29.02605 130
#> 8 3665.081 21.11620 121
#> 9 3402.094 44.22359 190
#> 10 4287.575 33.92261 163