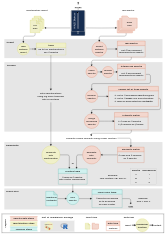
Dereplicate and Cherry-Pick Mass Spectrometry Spectra.
maldipickr 
- You are using the MALDI-TOF[^1] Biotyper to identify bacterial isolates
- You want to select representative isolates for further experiments
- You need fast and automated selection decisions that you can retrace
{maldipickr} provides documented and tested R functions that will help you dereplicate MALDI-TOF data and cherry-pick representative spectra of microbial isolates.
Check out the graphical overview.
{maldipickr}can use two approaches: from taxonomic identification reports (left) or from spectra data (right). Click on the thumbnail for a bigger version.
Installation
{maldipickr} is available on the CRAN and on GitHub.
To install the latest CRAN release, use the following command in R:
install.packages("maldipickr")
Or if you are using {renv}, use:
renv::install("maldipickr")
To install the development version, use the following command in R:
remotes::install_github("ClavelLab/maldipickr", build_vignettes = TRUE)
# or with renv::install("ClavelLab/maldipickr")
Usage
Start off with the Introduction to maldipickr for a quickstart. Otherwise, the comprehensive vignettes will walk you through the package functions and showcase how to:
- Import spectra data and identification reports from Bruker MALDI Biotyper into R.
- Process, dereplicate and cherry-pick representative spectra, from simple to complex design.
Troubleshoot and Contribute
Troubleshoot If something unexpected happened when using this package, please first search the current open or closed issues to look for similar problems. If you are the first, you are more than welcome to open a new issue using the “Bug report” template with a minimal reprex.
Contribute All contributions are welcome and the CONTRIBUTING.md documents how to participate. Please note that the {maldipickr} package is released with a Contributor Code of Conduct. By contributing to this project, you agree to abide by its terms.
Credits
Acknowledgements This R package is developed for spectra data generated by the Bruker MALDI Biotyper device. The {maldipickr} package is built from a suite of Rmarkdown files using the {fusen} package by Rochette S (2023). It relies on:
- the
{MALDIquant}package from Gibb & Strimmer (2012) for spectra functions - the work of Strejcek et al. (2018) for the dereplication procedure.
Disclaimer The developers of this package are part of the Clavel Lab and are not affiliated with the company Bruker, therefore this package is independent of the company and is distributed under the GPL-3.0 License. The hexagonal logo was created by Charlie Pauvert and uses the Atkinson Hyperlegible font font and a color palette generated at coolors.co.
References
- Gibb S & Strimmer K (2012). “MALDIquant: a versatile R package for the analysis of mass spectrometry data”. Bioinformatics 28, 2270-2271. https://doi.org/10.1093/bioinformatics/bts447.
- Rochette S (2023). “fusen: Build a Package from Rmarkdown Files”. <https://github.com/Thinkr-open/fusen.
- Strejcek M, Smrhova T, Junkova P & Uhlik O (2018). “Whole-Cell MALDI-TOF MS versus 16S rRNA Gene Analysis for Identification and Dereplication of Recurrent Bacterial Isolates.” Frontiers in Microbiology 9 https://doi.org/10.3389/fmicb.2018.01294.
[^1]: Matrix-Assisted Laser Desorption/Ionization-Time-Of-Flight (MALDI-TOF)