PHATE - Potential of Heat-Diffusion for Affinity-Based Transition Embedding.
phateR
This R package provides an implementation of the PHATE dimensionality reduction and visualization method.
For a thorough overview of the PHATE visualization method, please see the Nature Biotechnology publication.
For our Python and Matlab implementations, please see KrishnaswamyLab/PHATE.
Table of Contents
Installation
In order to use PHATE in R, you must also install the Python package.
If python or pip are not installed, you will need to install them. We recommend Miniconda3 to install Python and pip together, or otherwise you can install pip from https://pip.pypa.io/en/stable/installing/.
Installation from CRAN and PyPi
First install phate in Python by running the following code from a terminal:
pip install --user phate
Then install phateR from CRAN by running the following code in R:
install.packages("phateR")
Installation with devtools and reticulate
The development version of PHATE can be installed directly from R with devtools:
if (!suppressWarnings(require(devtools))) install.packages("devtools")
reticulate::py_install("phate", pip=TRUE)
devtools::install_github("KrishnaswamyLab/phateR")
Installation from source
The latest source version of PHATE can be accessed by running the following in a terminal:
git clone --recursive git://github.com/KrishnaswamyLab/PHATE.git
cd PHATE/Python
python setup.py install --user
cd ../phateR
R CMD INSTALL
If the phateR folder is empty, you have may forgotten to use the --recursive option for git clone. You can rectify this by running the following in a terminal:
cd PHATE
git submodule init
git submodule update
cd Python
python setup.py install --user
cd ../phateR
R CMD INSTALL
Quick Start
If you have loaded a data matrix data in R (cells on rows, genes on columns) you can run PHATE as follows:
library(phateR)
data_phate <- phate(data)
phateR accepts R matrices, Matrix sparse matrices, data.frames, and any other data type that can be converted to a matrix with the function as.matrix.
Tutorial
This is a basic example running phate on a highly branched example dataset that is included with the package. You can read a tutorial on running PHATE on single-cell RNA-seq at http://htmlpreview.github.io/?https://github.com/KrishnaswamyLab/phateR/blob/master/inst/examples/bonemarrow_tutorial.html or in inst/examples. Running this tutorial from start to finish should take approximately 3 minutes.
First, let’s load the tree data and examine it with PCA.
library(phateR)
#> Loading required package: Matrix
data(tree.data)
plot(prcomp(tree.data$data)$x, col=tree.data$branches)
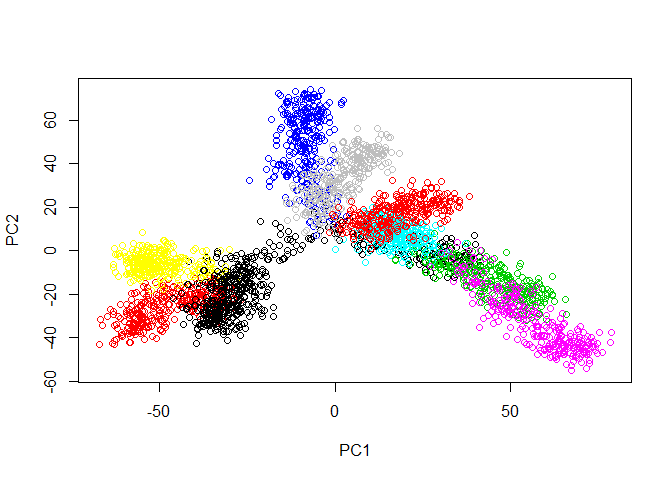
Now we run PHATE on the data. We’ll just go ahead and try with the default parameters.
# runs phate
tree.phate <- phate(tree.data$data)
summary(tree.phate)
#> PHATE embedding
#> k = 5, alpha = 40, t = auto
#> Data: (3000, 100)
#> Embedding: (3000, 2)
Let’s plot the results.
# plot embedding
palette(rainbow(10))
plot(tree.phate, col = tree.data$branches)
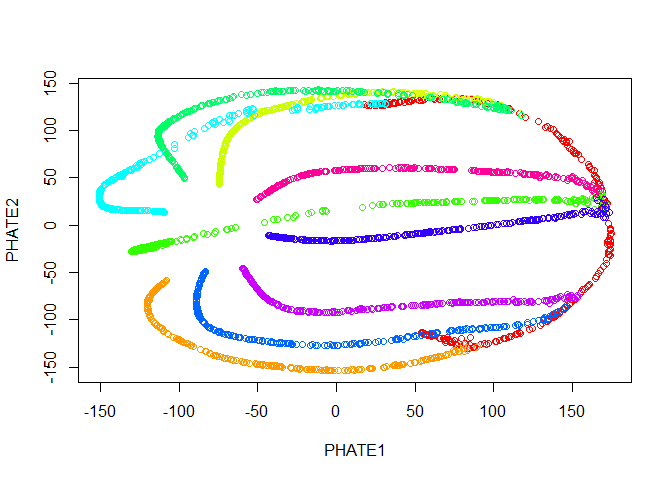
Good news! Our branches separate nicely. However, most of the interesting activity seems to be concentrated into one region of the plot - in this case we should try the square root potential instead by using gamma=0. We can also try increasing t to make the structure a little clearer - in this case, because synthetic data in unusually structured, we can use a very large value, like 120, but in biological data the automatic t selection is generally very close to ideal. Note here that if we pass our previous result in with the argument init, we won’t have to recompute the diffusion operator.
# runs phate with different parameters
tree.phate <- phate(tree.data$data, gamma=0, t=120, init=tree.phate)
# plot embedding
palette(rainbow(10))
plot(tree.phate, col = tree.data$branches)
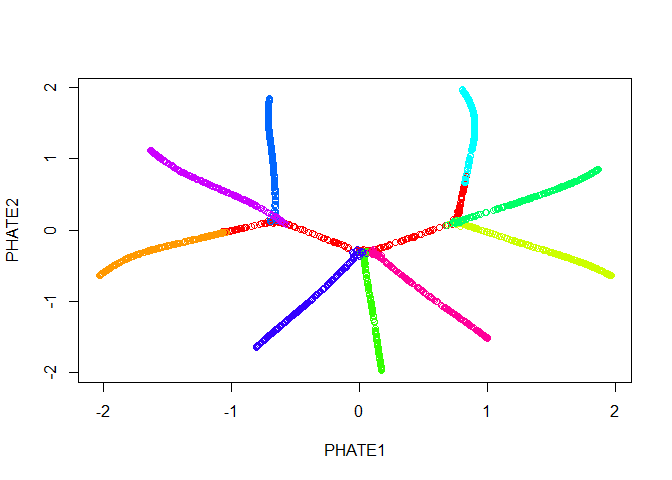
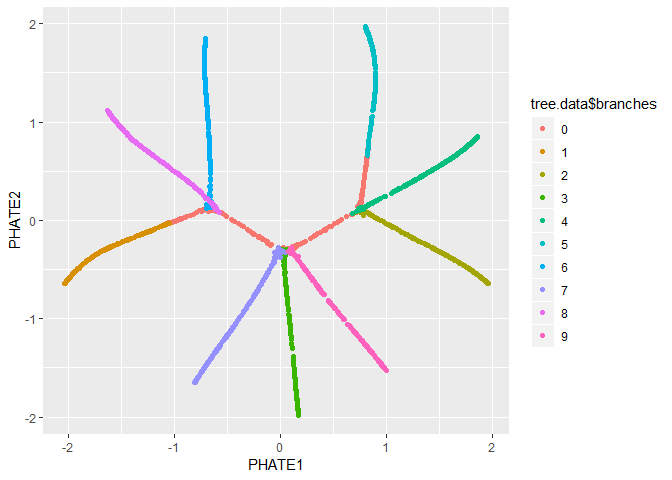
We can also pass the PHATE object directly to ggplot, if it is installed.
library(ggplot2)
#> Warning: package 'ggplot2' was built under R version 3.5.3
ggplot(tree.phate, aes(x=PHATE1, y=PHATE2, color=tree.data$branches)) +
geom_point()
Issues
FAQ
- Should genes (features) by rows or columns?
To be consistent with common dimensionality reductions such as PCA (stats::prcomp) and t-SNE (Rtsne::Rtsne), we require that cells (observations) be rows and genes (features) be columns of your input data.
- Can I run PHATE with Seurat?
PHATE was removed from Seurat in version 3. You can install a version of Seurat with RunPHATE included by following the instructions at https://github.com/satijalab/seurat/pull/1172#issuecomment-564782167.
- I have installed PHATE in Python, but phateR says it is not installed!
Check your reticulate::py_discover_config("phate") and compare it to the version of Python in which you installed PHATE (run which python and which pip in a terminal.) Chances are reticulate can’t find the right version of Python; you can fix this by adding the following line to your ~/.Renviron:
PATH=/path/to/my/python
You can read more about Renviron at https://CRAN.R-project.org/package=startup/vignettes/startup-intro.html.
Help
Please let us know of any issues at the GitHub repository. If you have any questions or require assistance using PHATE, please read the documentation at https://CRAN.R-project.org/package=phateR/phateR.pdf or by running help(phateR::phate) or contact us at https://krishnaswamylab.org/get-help.




